GenTegra GTR-STM
Non-Dilutive Saliva Sample Collection Products*
- Provides Non-Dilutive Saliva Sample Collection
- Improves Assay Sensitivity
- More Efficient SARS-CoV-2 RT-PCR Testing
- Maintains Viral RNA Sample Integrity During Heat Disinfection
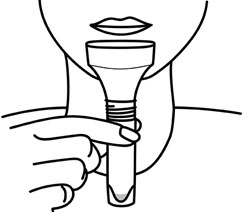
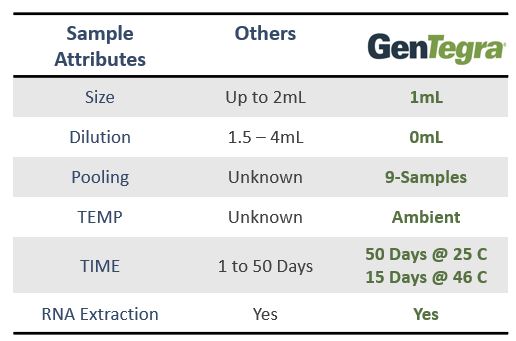
GenTegra introduces its COVID-19 saliva collection product to stabilize viral RNA. GTR-STM™ contains GenTegra’s novel Active Chemical Protection™ (ACP) formula, a proprietary non-dilutive dried media that stabilizes RNA. The saliva device for COVID-19 sample collection enhance patient comfort, convenience, and compliance.
GenTegra COVID-19 Product GTR-STM
GenTegra’s COVID-19 Kit, GTR-STM, saliva collection product can be used at remote testing sites or at-home. The collection tube contains GenTegra’s proprietary Active Chemical Protection formulation, a non-dilutive dry media. ACP facilitates detection of low virus levels that may be found in patients who are asymptomatic. Each saliva sample collected can be kept at ambient temperatures. The lab workflow is compatible with commonly used automated and manual extraction methods.